Professor Rolf Bjerkvig, a specialist in brain cancer research, tells us why guerrilla cells make the disease so hard to treat.
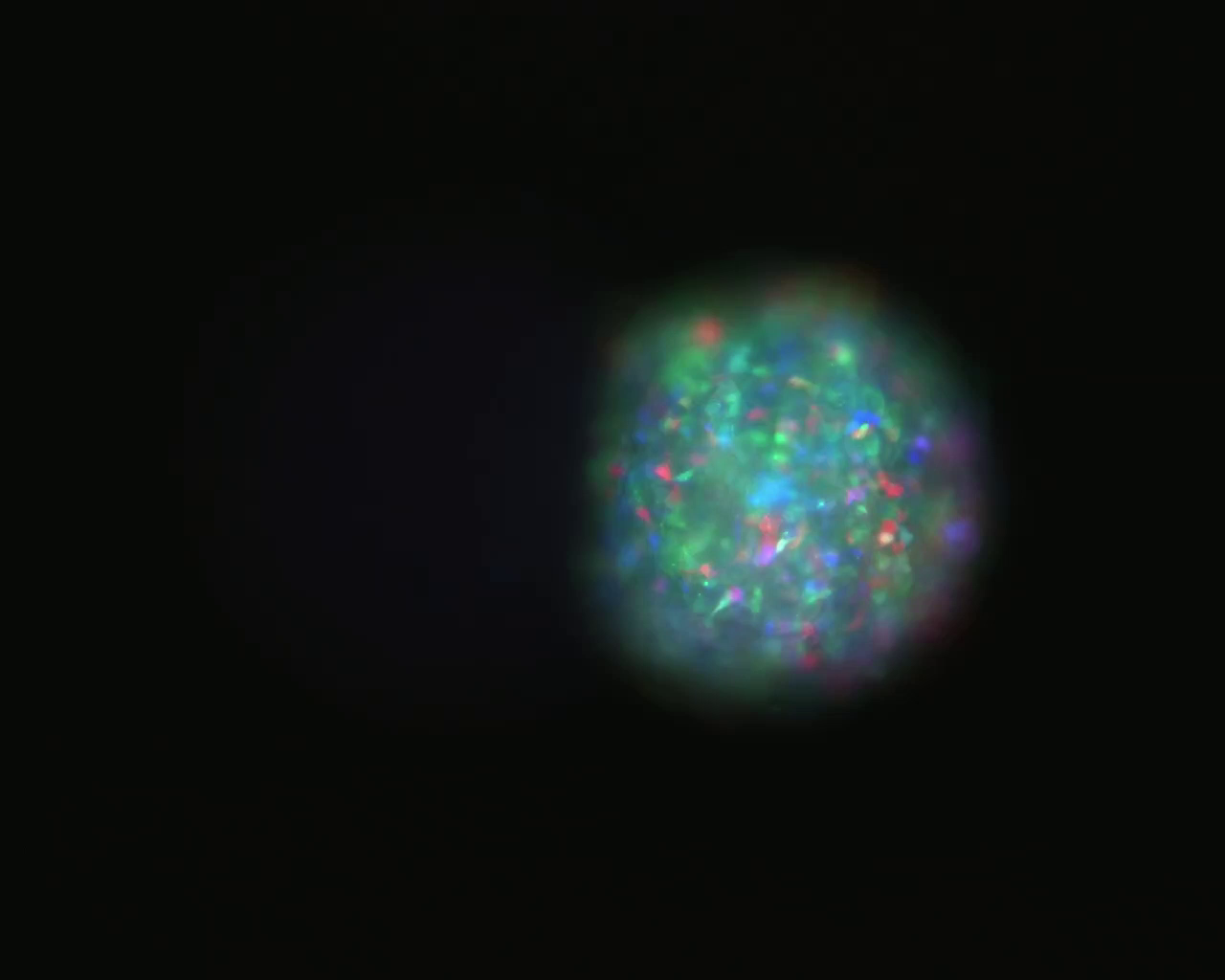
Brain tumours are hard to treat completely with surgery because they leave behind cells that invade the brain. In this video, which covers 72 hours, you can see cells breaking off from a tumour to invade other parts of the brain.
These cells are guerrilla cells in many ways – you can’t decipher them from normal cells. My research is that I try to understand why they are invading and how we can target these cells.
You can’t easily pick out these invasive cells in the human brain, so we grow mini-brains – organoids – in the lab. These organoids, with a size of 0.5mm, display the neurons and cellular components you have in a brain.
Then we take tumour biopsies from patients and barcode them with colour to see different tumour cell populations. We put them next to the mini-brains and then we can study their invasion into the brain.
What you see in the video is a brain tumour from a patient. You can see different cancer cells with different colours invading into the brain tissue. Brain cancers have lots of different tumour cell types, which makes them complex to treat. Therapies may kill one type of tumour cell but not all.
‘Brain cancers have lots of different types of tumour cells, which makes them complex to treat. Therapies may kill one type of tumour cell but not all.’ Professor Rolf Bjerkvig, Luxembourg Institute of Health
On the left side is the micro-brain but you can’t see it because it’s not stained. And with the video we can see the tumour cells migrating into the brain. We can also measure the speed of invasion and this gives us a unique opportunity to characterise the invasive cells and also to find out what kind of mechanism they are using for their migration.
Around 10 in 100,000 people develop brain tumours. For the most malignant type – glioblastoma – patients who are given the best therapy available live about 14-16 months after diagnosis. Brain tumours do not normally spread, like other cancers, but they stay in the brain. It’s therefore regarded as a local disease. The main problem is the location – the brain is the computer of the human body.
To get chemotherapeutics into the brain is difficult because the brain is protected by a so-called blood-brain barrier that prevents unwanted chemicals from the blood going into the brain. This barrier also prevents therapeutic substances from entering the brain. The brain thus protects the tumour from the therapy.
We have a large brain research centre in Norway and Luxembourg. At present we are working to find molecules so we can temporarily open the blood-brain barrier to make a delivery window of therapies into the brain.
There is a lot of ongoing research on brain cancer but there has been little progress on actually finding new therapies. One problem is that brain tumour research from the European Commission side has been very under-financed. There is a tendency to focus on more common tumour types that affect more people. Brain cancer is regarded as a rare disease. But in this context you are often competing for financing with neurological diseases and many other rare diseases.
In the private sector, pharma companies focus mostly on the development of blockbuster drugs that can work on millions of people rather than a couple of hundred thousand people. Brain cancer isn’t completely neglected but it is some way down in their portfolio.

Prof. Rolf Bjerkvig’s research group is looking at how to open the blood-brain barrier to allow treatments to reach brain tumours. Image Credit – Kim E. Andreassen, University of Bergen
There is at present a strong focus on personalised treatment initiatives. Many of these are based on sequencing the tumour genome, where the aim is to use this information to find the right therapy for the right patient. Unfortunately, transferring the knowledge gained from genetics into effective treatment has mostly been difficult. So can you shortcut this?
At Luxembourg Institute of Health, we are focusing on a personalised functional profiling (PFP) system where hundreds of microtumors are generated directly from a patient. These are then exposed, in an automated way, to a library of FDA approved drugs. In this way we can identify the most effective drugs that potentially will work. At the same time we do genomic profiling and then we can correlate the drugs to the genomic profile.
We have seen promising effects using this approach but we need to increase the number of patients and then we can start to make statistical analysis. That’s why we need support for our PFP centre. It should, however be recognised that many of these drugs wouldn’t cross the blood-brain barrier. So in many instances this needs to be combined with the strategies for delivery – how to deliver this to the brain.
Prof. Bjerkvig is head of the oncology department at Luxembourg Institute of Health and a professor anatomy and cell biology at the University of Bergen in Norway. He led the EU-funded project ANGIOTARGETING.
As told to Joanna Roberts. This account has been edited and condensed. The views expressed in the article are those of the interviewee and do not necessarily reflect the position of the European Commission.
Originally published on Horizon.